| The hepatitis B
virus consists of a core containing DNA and a DNA polymerase enzyme needed for
virus replication. The core of the virus is surrounded by surface protein . The virus, also called a Dane particle,
and an excess of its surface protein (known as hepatitis B surface antigen)
circulate in the blood. Humans are the only source of infection.
|
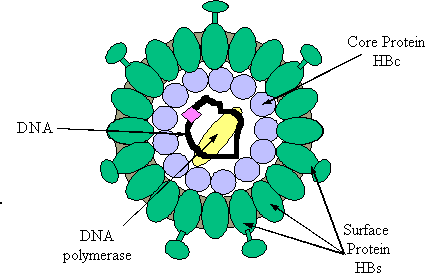
| . Hepatitis B surface antigen
(HBsAg) is a protein which makes up part of the viral envelope. Hepatitis B core
antigen (HBcAg) is a protein which makes up the capsid or core part of the virus
(found in the liver but not in blood). Hepatitis B e antigen (HBeAg) is part of
the HBcAg which can be found in the blood and indicates
infectivity. |
| Hepatitis B
infection affects 300 million people and is one of the most common causes of
chronic liver disease and hepatocellular carcinoma world-wide.
|
| Hepatitis B may
cause an acute viral hepatitis; however, the acute infection is often
asymptomatic, particularly when acquired at birth. Many individuals with chronic
hepatitis B are also asymptomatic. Chronic hepatitis, associated with elevated
serum transaminases, may occur and can lead to cirrhosis, usually after decades
of infection |
The risk of
progression to chronic liver disease depends on the source of infection Vertical transmission, from mother to child in the perinatal period,
is the most common cause of infection world-wide and carries the highest risk.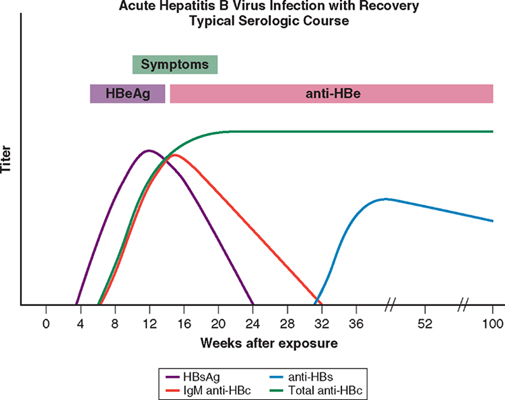 |
SOURCE OF HEPATITIS B
INFECTION AND RISK OF CHRONIC INFECTION
|
| Route of transmission |
Risk of chronic infection |
| Horizontal
transmission |
10% |
| Injection drug use |
|
| Infected unscreened blood
products |
|
| Tattoos/acupuncture
needles |
|
| Sexual (homosexual and
heterosexual) |
|
| Vertical
transmission |
90% |
| HbsAg-positive
mother |
|
| There is an initial
immunotolerant phase with high levels of virus and normal liver biochemistry. An
immunological response to the virus then occurs, with elevation in serum
transaminases which causes liver damage: chronic hepatitis. If this response is
sustained over many years and viral clearance does not occur promptly, chronic
hepatitis may result in cirrhosis. In individuals where the immunological
response is successful, viral load falls, HBe antibody develops and there is no
further liver damage. Some individuals may subsequently develop HBV-DNA mutants,
which escape from immune regulation, and viral load again rises with further
chronic hepatitis. Mutations in the core protein result in the virus's inability
to secrete HBe antigen despite high levels of viral replication; such
individuals have HBeAg-negative chronic hepatitis. (ALT = alanine
aminotransferase; AST = aspartate aminotransferase) |
| (HBsAg = hepatitis
B surface antigen; anti-HBs = antibody to HBsAg; HBeAg = hepatitis B e antigen;
anti-HBe = antibody to HBeAg; anti-HBc = antibody to hepatitis B core
antigen) |
INTERPRETATION OF MAIN
INVESTIGATIONS USED IN THE SEROLOGICAL DIAGNOSIS OF HEPATITIS B VIRUS
INFECTION
|
| |
|
Anti-HBc |
|
| Interpretation |
HBsAg |
IgM |
IgG |
Anti-HBs |
| Incubation
period |
+ |
+ |
- |
- |
| Acute
hepatitis |
| Early |
+ |
+ |
- |
- |
| Established |
+ |
+ |
+ |
- |
| Established
(occasional) |
- |
+ |
+ |
- |
| Convalescence |
| (3-6 months) |
- |
± |
+ |
± |
| (6-9 months) |
- |
- |
+ |
+ |
| Post-infection |
| > 1 year |
- |
- |
+ |
+ |
| Uncertain |
- |
- |
+ |
- |
| Chronic
infection |
| Usual |
+ |
- |
+ |
- |
| Occasional |
- |
- |
+ |
- |
| Immunisation without
infection |
- |
- |
- |
+ |
|
+ positive; -
negative; ± present at low titre or absent. |
| HBV contains
several antigens to which infected persons can make immune responses these antigens and their antibodies are
important in identifying HBV infection |
| In acute infection
the hepatitis B surface antigen (HBsAg) is a reliable marker of HBV infection,
and a negative test for HBsAg makes HBV infection very unlikely but not
impossible . HBsAg appears in the blood late in the
incubation period and before the prodromal phase of acute type B hepatitis; it
may be present for only a few days, disappearing even before jaundice has
developed, but usually lasts for 3-4 weeks and can persist for up to 5 months.
|
| Antibody to HBsAg
(anti-HBs) usually appears after about 3-6 months and persists for many years or
perhaps permanently. Anti-HBs implies either a previous infection, in which case
anti-HBc (see below) is usually also present, or previous vaccination when
anti-HBc is not present. |
| The hepatitis B
core antigen (HBcAg) is not found in the blood, but antibody to it (anti-HBc)
appears early in the illness and rapidly reaches a high titre which then
subsides gradually but persists. Anti-HBc is initially of IgM type with IgG
antibody appearing later. Anti-HBc (IgM) can sometimes reveal an acute HBV
infection when the HBsAg has disappeared and before anti-HBs has developed (and |
| The hepatitis B e
antigen (HBeAg) appears only transiently at the outset of the illness and is
followed by the production of antibody (anti-HBe). The HBeAg reflects active
replication of the virus in the liver. The persistence of HBsAg for longer than
6 months indicates chronic infection. |
| Chronic HBV
infection (see below) is marked by the presence of HBsAg and anti-HBc (IgG) in
the blood. Usually, HBeAg or anti-HBe is also present; HBeAg indicates continued
active replication of the virus in the liver while anti-HBe implies that
replication is occurring at a much lower level or that HBV-DNA has become
integrated into host hepatocyte DNA. |
| HBV-DNA encodes four proteins: a DNA
polymerase needed for viral replication (P), a surface protein (S), a core
protein (C) and an X protein. The pre-C and C regions encode a core protein and
an e antigen. Although mutations in the hepatitis B virus are frequent
occurrences, certain mutations have important clinical effects. Pre-C encodes a
signal sequence needed for the C protein to be secreted from the liver cell into
serum as e antigen. A mutation in the pre-core region leads to a failure of
secretion of e antigen into serum and so individuals have high levels of viral
production but no detectable e antigen in the serum. Mutations can also occur in
the surface protein and may lead to the failure of vaccination (surface
antibodies produced against native S protein) to prevent infection. Mutations
also occur in the DNA polymerase during antiviral treatment with
lamivudine. |
| HBV-DNA can be
measured by polymerase chain reaction (PCR) in the blood. Viral loads are
usually in excess of 105 copies/ml in the presence of active viral
replication, as indicated by the presence of e antigen. In contrast, in those
with low viral replication, HBsAg- and anti-HBe-positive, viral loads are less
than 105 copies/ml. The exception is in patients who have a mutation
in the pre-core protein, which means they cannot secrete e antigen into serum . Such individuals will be anti-HBe-positive
but have a high viral load and often evidence of chronic hepatitis. These
mutations are common in the Far East and those affected are classified as having
e antigen-negative chronic hepatitis. They respond differently to antiviral
drugs from those with classical e antigen-positive chronic hepatitis.
|
| Measurement of
viral load is important in monitoring antiviral therapy and identifying patients
with pre-core mutants. Specific HBV genotypes can also be identified using PCR.
Genotypes B and C appear to have more aggressive disease that responds less well
to antiviral therapy. |
| Treatment is
supportive with monitoring for acute liver failure, which occurs in less than 1%
of cases. |
| Treatments are
still limited, with no drug able to eradicate hepatitis B infection completely.
The indication for treatment is a high viral load in the presence of active
hepatitis, as demonstrated by elevated serum transaminases and/or histological
evidence of inflammation. |
| This is most
effective in selected patients with a low viral load and serum transaminases
greater than twice the upper limit of normal in whom it acts by augmenting a
native immune response. In HBeAg-positive chronic hepatitis 33% lose e antigen
after 4-6 months of treatment compared to 12% of controls. Response rates are
lower in HBeAg-negative chronic hepatitis, even when patients are given longer
courses of treatment. Interferon is contraindicated in the presence of cirrhosis
as it may cause a flare in serum transaminases and precipitate liver failure.
Longer-acting pegylated interferons which can be given once weekly have been
evaluated in both HBeAg-positive and HBeAg-negative chronic hepatitis . Other antiviral therapies are required because many patients with
chronic hepatitis B have high levels of viraemia and/or low transaminase levels
and are not therefore candidates for interferon. |
PEGYLATED INTERFERONS IN
CHRONIC HEPATITIS B INFECTION
|
| 'In HBeAg-positive
chronic hepatitis treatment with pegylated interferon for 6 months eliminates
HBeAg in 35%, and normalises liver biochemistry in 25% of patients. In
HBeAg-negative chronic hepatitis treatment with pegylated interferon for 12
months leads to normal liver biochemistry in 60%, and sustained suppression of
hepatitis B virus load below 400 copies/ml in 20% of
patients.' |
|

